Updated December 14th, 2020
On December 11, 2020, the FDA approved the first emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine. The Vaccine can be distributed to individuals who are 16 years of age or older. The vaccine is to be administered to frontline healthcare providers, first responders and those at high risk of complications due to COVID-19. It is currently being predicted that the vaccine will not be available to the common majority for several months due to limited supply and demand.
Most are concerned with the side effects of the vaccine and what we can expect. Check out this fact sheet provided by the FDA for more information.
Will we see a COVID-19 Vaccine before 2021?
The number of Coronavirus (COVID-19) cases is rising once again in the United States and some states are struggling with hospitals being at capacity due to more severe cases. Some say that the surge that many healthcare providers planned for in April 2020 is now taking place. With talks of a national shutdown right before the holidays, many have been discussing a safe and effective vaccine that can help save lives and restore normalcy once again. We are currently in a very critical time where the number of cases is rising and the realizations of an FDA-approved vaccine being readily available is edging closer and closer with every passing day.
The Latest News on a Possible COVID-19 Vaccine
There has been so much speculation around COVID-19 research, including potential vaccines. On December 1, 2020, the ACIP recommended that once an FDA approved vaccine is available, the vaccination in the initial phase of the COVID-19 vaccination program (Phase 1a) should be offered to both 1) healthcare personnel and 2) residents of long-term care facilities.
The latest news has been released, reporting that the Food and Drug Administration (FDA) announced a scheduled meeting on December 10, 2020, for its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to discuss the request for emergency use authorization (EUA) for a COVID-19 vaccine from Moderna Inc. and Pfizer. Pfizer is already being administered in Great Britain and is currently the only vaccine to be fully tested in and the process of becoming readily available in other countries. The United States is planning on purchasing the Pfizer vaccines once it is approved by the FDA. The United States is treading lightly to ensure that the vaccine is safe and effective in its early stages of development. Unfortunately, vaccines can have a long period of time before showing any side-effects so there is a significant amount of risk in fast-tracking any vaccine. There is some hesitation due to the uncharted waters of long-lasting effects the COVID-19 vaccine may cause in the future but the duty to save the lives of those being infected is the driving force between the risks of advanced medicine or doing nothing.
According to the Press Release from the FDA, with a strong focus on full transparency with the public regarding a COVID-19 Vaccine, they intend to issue a Federal Register notice as soon as possible with details of the meeting. Details will include information about a public docket that will allow the public to comments that will later be reviewed by the FDA. The FDA also intends to livestream the VRBPAC meeting on the agency’s social media channels; the meeting will also be webcast from the FDA website.
What Do We Know
At this time, there is currently no authorized or approved vaccine to prevent coronavirus (COVID-19). Our hope is that COVID-19 vaccines will help prevent the spread of the virus that can be very dangerous and even fatal. Though we are not sure how well the vaccine, specifically for COVID-19 will work, it has been proven that authorized or approved vaccines help reduce the risk of disease by working with the body’s natural defenses to safely develop an immunity to a targeted disease.
To better understand how COVID-19 vaccines work, we have to understand our body’s natural response to a virus such as COVID-19. The COVID-19 virus can be transmitted in multiple ways including water droplets and airborne particles. Once the virus enters the body, it begins to multiply and attack causing an infection and leads to showing symptoms such as a fever, coughing, and shortness of breath.
Antibodies and their Role in Fighting Infection
According to an article from the CDC, our immune system uses several tools to fight infection. Blood contains red cells, which carry oxygen to tissues and organs, and white or immune cells, which fight infection. Different types of white blood cells fight infection in different ways:
- Macrophagesare white blood cells that swallow up and digest germs and dead or dying cells. The macrophages leave behind parts of the invading germs called antigens. The body identifies antigens as dangerous and stimulates antibodies to attack them.
- B-lymphocytes are defensive white blood cells. They produce antibodies that attack the pieces of the virus left behind by the macrophages.
- T-lymphocytesare another type of defensive white blood cell. They attack cells in the body that have already been infected.
Once the human body is infected with a virus, like COVID-19, it can take several days or weeks to naturally fight off the infection. After the infection, the body keeps memory cells called T-lymphocytes, which will identify the virus if you are exposed again. When the familiar antigens are detected, B-lymphocytes produce antibodies to attack them. According to the CDC, Experts are still learning how long these memory cells protect a person against the virus that causes COVID-19.
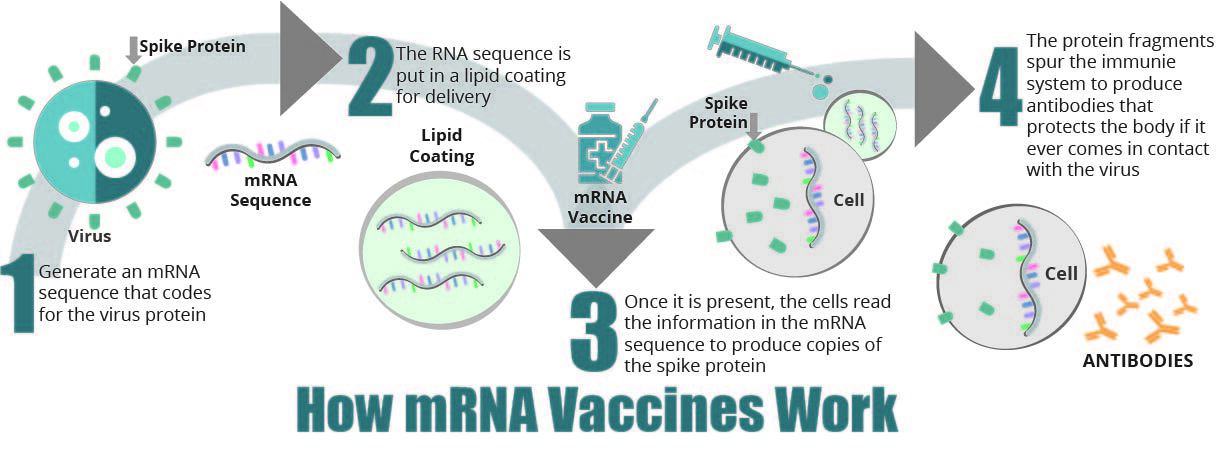
How COVID-19 Vaccines Work
Research has shown that the new COVID-19 vaccines help develop immunity to the coronavirus without directly infecting anyone with the illness. This process can take time and it does not guarantee that the recipient e will not contract the virus before the vaccine has enough time to provide protection.
Different types of vaccines work in different ways to offer protection, but with all types of vaccines, the body is left with a supply of “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight that virus in the future.
Types of Vaccines
The CDC provided the following types of COVID-19 vaccines that are or soon will be undergoing large-scale (Phase 3) clinical trials in the United States. Below is a description of how each type of vaccine prompts our bodies to recognize and protect us from the virus that causes COVID-19. None of these vaccines can give you COVID-19.
- mRNA vaccines contain material from the virus that causes COVID-19 which gives our cells instructions for how to make a harmless protein that is unique to the virus. After our cells make copies of the protein, they destroy the genetic material from the vaccine. Our bodies recognize that the protein should not be there and build T-lymphocytes and B-lymphocytes that will remember how to fight the virus that causes COVID-19 if we are infected in the future.
- Protein subunit vaccines include harmless pieces (proteins) of the virus that cause COVID-19 instead of the entire germ. Once vaccinated, our immune system recognizes that the proteins don’t belong in the body and begins making T-lymphocytes and antibodies. If we are ever infected in the future, memory cells will recognize and fight the virus.
- Vector vaccinescontain a weakened version of a live virus—a different virus than the one that causes COVID-19—that has genetic material from the virus that causes COVID-19 inserted in it (this is called a viral vector). Once the viral vector is inside our cells, the genetic material gives cells instructions to make a protein that is unique to the virus that causes COVID-19. Using these instructions, our cells make copies of the protein. This prompts our bodies to build T-lymphocytes and B-lymphocytes that will remember how to fight that virus if we are infected in the future.
Vaccine Trials that are Making Strides
As of November 24, 2020, large-scale (Phase 3) clinical trials are in progress or being planned for five COVID-19 vaccines in the United States: The Moderna Vaccine and the Pfizer Vaccine will be discussed with the FDA on December 10, 2020, for potential emergency authorization.
- AstraZeneca’s COVID-19 vaccine
- Janssen’s COVID-19 vaccine
- Moderna’s COVID-19 vaccine
- Novavax’s COVID-19 vaccine
- Pfizer’s COVID-19 vaccine
There are many people following the media closely to gather information about a potential vaccine that is safe and effective. As time continues to pass and COVID-19 cases rise, we are all hoping that experts are able to provide a solution to a pandemic that has affected humanity on a global scale. Once a vaccine is approved, the first to receive the vaccine will be healthcare providers working on the front lines. As of December 1, 2020, approximately 245,000 COVID-19 cases and 858 COVID-19-associated deaths had been reported among U.S. health care personnel.
Sited Links:
https://www.cdc.gov/mmwr/volumes/69/wr/mm6949e1.htm
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect.html
ACIP Resources: Meeting agendas, minutes, live meetings, and presentation slides
Learn more about ACIP’s Ethical Principles for Allocating Initial Supplies of COVID-19 Vaccine.